BRAZIL – 2021/11/26: On this photo illustration a F. HoffmannLa Roche AG logo is seen on a screen and a hand holding a pill. (Photo Illustration by Rafael Henrique/SOPA Images/LightRocket via Getty Images)
Sopa Images | Lightrocket | Getty Images
Swiss pharmaceuticals giant Roche is about to amass anti-obesity drug developer Carmot Therapeutics, becoming the newest company to try to unseat Novo Nordisk and Eli Lilly’s dominance inside the global weight reduction drugs market.
Under the deal terms, Carmot’s equity holders will receive $2.7 billion in money on the transaction’s close and will pocket as much as an additional $400 million, depending on reaching certain milestones.
The U.S. takeover goal’s early stage technology could help crack highly prized oral obesity treatments, Roche Pharmaceuticals CEO Teresa Graham said Monday, however it could also be several years before the drugs are widely available.
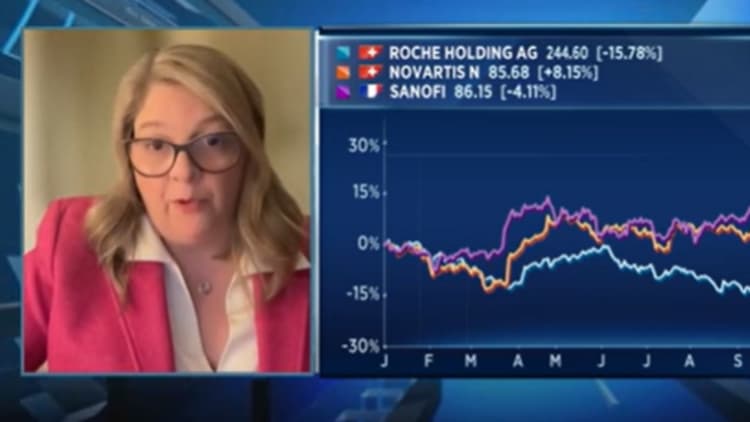
“These assets are all relatively early stage, so we might expect the 2030+ time-frame is after we’ll actually give you the chance to bring these products to market,” Graham told CNBC’s Julianna Tatelbaum.
The deal will provide Roche access to Carmot’s current research and development portfolio, including all clinical and preclinical assets.
Shares of the Swiss company, which have been within the doldrums this yr, were up 2.25% after news of the acquisition deal.
California-based Carmot’s most promising drug candidate, a once-weekly injection called CT-388, belongs to a category generally known as dual GLP-1/GIP receptor agonists — that are similar to those utilized by Eli Lilly‘s Mounjaro, or Zepbound, and mimic a hormone typically released into the body after eating.
After encouraging Phase 1 trial results, the drug is now attributable to be tested on humans within the second of three trial stages, Roche said in a statement.
Carmot’s once-daily oral candidate generally known as CT-996, which is is currently undergoing Phase 1 trials, could help differentiate Roche in an increasingly crowded obesity drugs market.
“The products that we’re acquiring in 996 has some interesting data to it,” Graham said.
“I do think that we are going to work out learn how to deliver these drugs orally; it’s only a matter of time,” she added.
Obesity pill trials ramp up
A series of pharmaceutical corporations are currently trialing oral obesity treatments within the hopes of improving patient accessibility. Astra Zeneca last month announced that it could pay as much as $2 billion for the rights to an experimental pill from China’s Eccogene, in accordance with Reuters.
Nevertheless, analysts have expressed caution over the efficacy of such treatments, and Pfizer dropped its plans for a twice-weekly pill last week after recording a spike in unintended effects.
It comes as latest entrants pile into the worldwide obesity market — estimated to be price $200 billion inside the subsequent decade — while existing heavyweights Novo Nordisk and Eli Lilly struggle to maintain up with soaring demand.
Roche was amongst one in all the primary drugmakers to work on GLP-1 treatments greater than a decade ago, but halted its initial trials after patients dropped out. Graham said Monday that now could be a “great time” to be reentering the market.
“We’ve an enormous amount of experience to bring to bear from the diabetes franchise in diagnostics which I believe will probably be a very exciting partnership,” Graham said. “The acquisition of Carmont only serves to bolster what’s already quite an exciting and diverse pipeline.”










