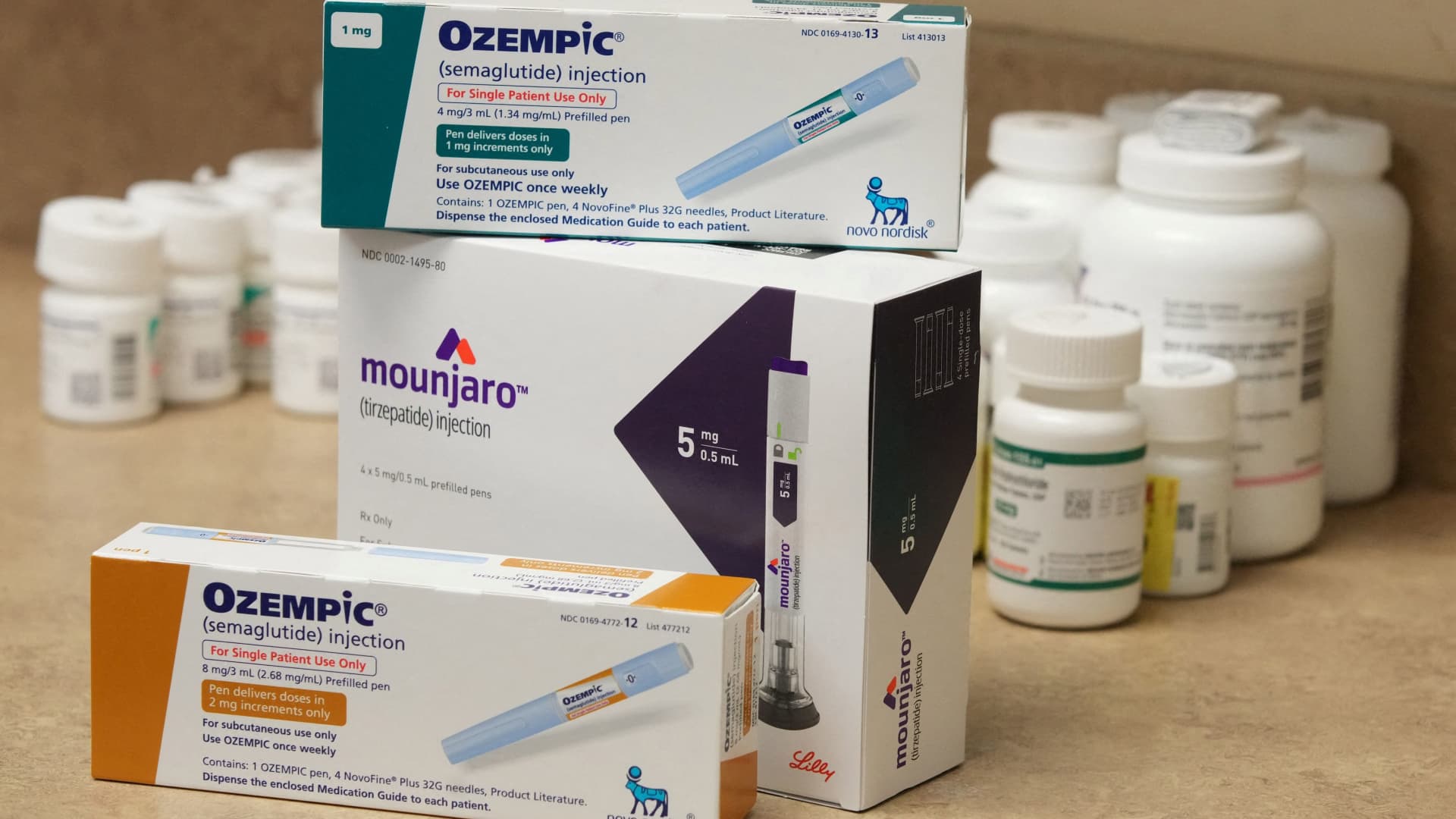
While there was loads of buzz about how successful patients have been in reducing weight while taking a latest class of obesity medications, there may be a growing realization that when people stop taking these pricey drugs, the kilos can creep back on. There was a frank discussion of this on Eli Lilly’s first-quarter earnings conference call on Thursday. “My expectation is many patients may try coming off the drug completely to see what happens,” said Daniel Skovronsky, Lilly’s chief scientific and medical officer, on the decision. “Perhaps some shall be successful maintaining their weight, but a lot of them will probably experience some regression of their weight back towards baseline. And this might prompt them to return back on the drug. That is probably natural, and we will expect that.” Lilly manufactures tirzepatide, which is sold under the brand name Mounjaro as a kind 2 diabetes treatment. Clinical trials are underway for its use as a weight reduction treatment, and the Food and Drug Administration is widely expected to approve it later this yr. But when it gets the FDA’s nod, the corporate’s work won’t be over. Lilly will need to alter the way in which people — and insurers — view weight reduction. “On the industrial side, as we launch into the chronic weight management marketplace for tirzepatide, we’ll be very upfront with payers and health-care professionals and consumers that this can be a chronic disease and a chronic medication that should be adhered to long-term,” said Michael Mason, who heads up Lilly’s diabetes unit, on Thursday. It is the chronic nature of obesity that may make these drugs so profitable, analysts have said. Some have predicted tirzepatide will develop into a blockbuster with annual sales of as much as $100 billion by 2035 , by Bank of America’s forecasts. But there may be a growing chorus focused on who will bear the burden of those costs. If medical insurance firms beat back against it, the lofty sales forecasts won’t materialize. Already some private insurers that cover the drug will accomplish that just for a set time frame. It’s even worse news under Medicare. The federal medical insurance program for the elderly and long-term disabled is banned from covering weight reduction medications, although it should fund bariatric surgery. ‘It’s hard to assume’ For now, each Eli Lilly and the analysts who cover the corporate remain optimistic. Lilly expects medications to develop into the usual of look after obesity, and analysts are ratcheting up their near-term sales forecasts for tirzepatide based solely on the trends seen in Lilly’s first quarter with type 2 diabetes patients. “It’s hard to assume by the top of this decade, that everybody doesn’t just accept that pharmacologic treatment for obese and obesity needs to be the usual of care and it should save the health-care system trillions of dollars over time,” CEO David Ricks told analysts. “So you realize, that is our position and we’d like to fight for that position.” To make this case, Lilly and other pharmaceutical firms within the space, including Wegovy manufacturer Novo Nordisk , are pointing to the belief that treating obesity and obese will reduce the occurrence of other comorbidities, producing cost savings for the system. This includes conditions like sleep apnea, heart disease and cancer, amongst other chronic conditions. The businesses are conducting trials that hope to prove this argument but it should take a while to collect enough information to document it. Specifically, Novo and Lilly are whether these drugs can prevent stroke or heart attacks in patients with diabetes. Also they are monitoring the impact on sleep apnea and osteoarthritis within the knee. As research continues, there are also other variations of those drugs being studied. This class of medicines are sometimes called GLP-1 drugs because they mimic incretin hormones within the body referred to as glucagon-like peptide-1. Wegovy uses semaglutide in a once every week injection. This same drug can be sold by Novo because the brand Ozempic for diabetes treatment. Mounjaro features a second incretin hormone, glucose-dependent insulinotropic polypeptide, or GIP. It also is run by injection once every week. Each firms, in addition to others resembling Pfizer , Amgen , and Viking , are studying other incretin combos, including ones that could possibly be administered orally. The present state of play Novo and Lilly have the sting. Novo was the primary to win approval for weight reduction, but Lilly has been shown to be barely more practical in clinical trials . Each drugs suppress the appetite of patients and slow how quickly food passes through the gastric system. The news was pretty upbeat for Mounjaro on Thursday. “Mounjaro was the highlight of the quarter with sales coming in ahead of expectations on higher [average selling prices] and with patient access continuing to tick up,” wrote JPMorgan analyst Chris Schott, in a research note. Schott expects the trend to proceed within the second quarter and construct further within the second half of the yr. “And more broadly on the story, LLY continues to represent our favourite pick within the group as we see meaningful upside to Street estimates for Mounjaro and LLY’s broader incretin portfolio over time in addition to a positive setup on the stock heading into quite a lot of essential catalysts in 2023 and early 2024,” he said, referring to Lilly’s next-generation incretin drugs and data on its Alzheimer’s treatment, amongst other aspects. Several firms increased their price targets for Lilly shares within the wake of this week’s results. Lilly shares are up 8% yr to this point. That is on top of a 32% gain the stock logged in 2022. On Friday, shares set a fresh 52-week high of $404.31. Credit Suisse analyst Trung Huynh expects Lilly shares could hit $420 in the approaching yr, which is about 6% above where shares closed Friday. Huynh increased his goal as a consequence of higher expectations for Mounjaro sales this yr. Schott has a Dec. 2023 price goal of $430. LLY 1Y mountain Eli Lilly shares hit a fresh 52-week high on Friday In the primary quarter, Mounjaro tallied $568.5 million in sales, 8% above consensus estimates. Huynh boosted his prediction for the drug’s sales this yr to $3.7 billion from an prior estimate of $2.7 billion. One factor driving the drug’s use is a rise in health insurers covering the drug, which may cost about $12,000 a yr. As of April 1, about 60% of insurers are covering Mounjaro for type 2 diabetes, up from 40% of insurers at the top of last yr. Throughout the first quarter, Morgan Stanley analyst Terence Flynn said Mounjaro’s gross-to-net improved to $243 per script from $136 per script as more insurance firms added the drug to their formularies. Still, there could possibly be a hiccup within the Mounjaro’s growth trajectory. In June, coupons that help patients cover the drug’s cost will expire. With the coupon, type 2 diabetes patients have been paying just $25 a month for the drug. The expiration could force some patients to stop taking it, or slow additional patients from making the move to it from other treatments. For this reason it is actually essential to appreciate it continues to be very early days with this drug. ‘On the precipice’ “We’re principally just on the precipice of this market taking off,” Mizuho analyst Jared Holz said on CNBC’s “The Exchange” on Friday. He noted that although investors have been talking about this for some time, the ramp up in revenue continues to be to return. Novo Nordisk’s experience with Wegovy has been encouraging for what could occur once tirzepatide gains approval for obesity treatment. In mid-April, Credit Suisse upgraded Novo Nordisk , citing the strength it was seeing in its relaunch of Wegovy after manufacturing issues that were causing shortages of the drug were resolved. Nonetheless, Holz said the present usage of those medications almost makes them “vanity” drugs. “From the numbers that you’ve got seen, I’m not likely sure that the folks that are really most suited to the drugs are literally getting them,” he said. Holz is amongst those that have expressed concerns that medical insurance firms may resist adoption. “$48 billion is a monster number,” Holz said, referring to an annual sales estimate that Bank of America has floated for future Mounjaro sales. “… It just looks as if in some unspecified time in the future, the payers are going to, you realize, obviously be impacted.” —CNBC’s Michael Bloom contributed to this report.
CEO who used ChatGPT to refer to business icons: ‘Advice was so good’
Joanna Stober, Midi Health CEO and co-founder, has never had a possibility to run her business plans past legendary enterprise...