MRI image of brain showing area of Alzheimer patient.
Getty Images
The Food and Drug Administration on Friday granted accelerated approval for the Alzheimer’s drug lecanemab, the second treatment from Biogen and its Japanese partner Eisai to receive an early green light in lower than two years.
The FDA’s approval comes after clinical trial results published in November indicated that lecanemab slows cognitive decline somewhat in individuals with mild impairment on account of Alzheimer’s disease, however the treatment also carries risks of brain swelling and bleeding.
Eisai, which led the event of lecanemab, is pricing the treatment at $26,500 per 12 months within the U.S. It is going to be sold under the name Leqembi.
The FDA can speed up approval of a drug to quickly bring it to market if it’s expected to assist patients affected by serious conditions greater than what’s currently available. Biogen and Eisai applied for accelerated approval in July.
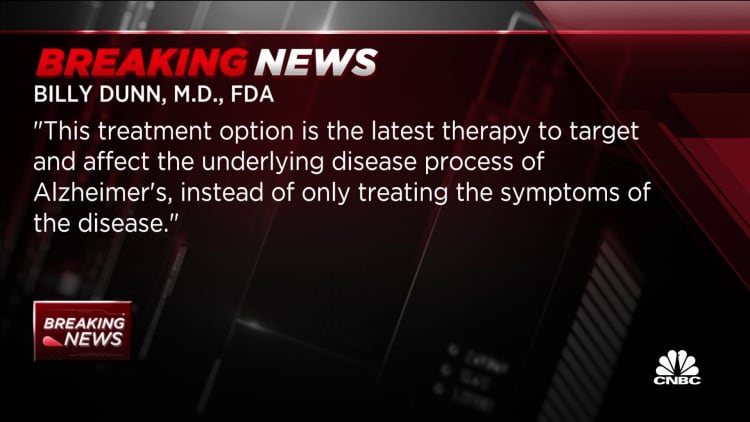
“Alzheimer’s disease immeasurably incapacitates the lives of those who are suffering from it and has devastating effects on their family members,” said Dr. Billy Dunn, director of the FDA’s neuroscience division, in a press release. “This treatment option is the most recent therapy to focus on and affect the underlying disease means of Alzheimer’s, as a substitute of only treating the symptoms of the disease.”
Greater than 6.5 million people within the U.S. suffer from Alzheimer’s. The irreversible disease destroys memory, considering skills, and eventually the power to perform easy tasks.
The choice on lecanameb comes after Congress issued a scathing report last week about how the FDA handled the controversial approval of one other Alzheimer’s drug developed by Biogen and Eisai, called Aduhelm. The 2021 approval of that treatment, which experts said didn’t show a transparent clinical profit, was “rife with irregularities,” in response to the report.
The congressional report said the “FDA must take swift motion to be sure that its processes for reviewing future Alzheimer’s disease treatments don’t result in the identical doubts concerning the integrity of FDA’s review.”
Modestly slows disease
Lecanemab is a monoclonal antibody that targets a protein called amyloid which builds up on the brain in individuals with Alzheimer’s. The antibody is run intravenously every two weeks in doses determined by a patient’s body weight with 10 milligrams given per kilogram.
The FDA approved lecanemab based on the reduction of amyloid plaque observed in clinical trial participants who received the treatment, in response to a press release from the agency. Participants who didn’t receive the treatment, the placebo arm, had no reduction in amyloid plaque.
The clinical trial results, published within the Latest England Journal of Medicine, found that cognitive decline was 27% slower over 18 months in individuals who received lecanemab compared with those that didn’t receive the treatment. The study was funded by Biogen and Eisai.
Cognitive decline was measured using a system called the clinical dementia rating, which is an 18-point scale with the next rating indicating a greater level of impairment. It measures cognitive functions corresponding to memory, judgement and problem solving.
Alzheimer’s disease progressed 1.21 points on average within the group that received lecanemab compared with 1.66 points within the group that didn’t receive the treatment, a modest difference of 0.45 points.
Nearly 1,800 people ages 50 to 90 years old with early Alzheimer’s participated within the trial, about half of whom received lecanemab and half of whom didn’t.
Safety concerns
Though lecanemab may slow cognitive decline somewhat, the treatment also carries risks.
Nearly 13% of those that received lecanemab developed brain swelling compared with about 2% within the group that did not receive the treatment. Nevertheless, most of those cases were mild to moderate in severity, didn’t cause symptoms, and typically resolved inside 4 months.
About 3% of patients who received lecanemab had more serious brain swelling with symptoms that included headache, visual disturbance and confusion.
About 17% of those that received lecanemab had brain bleeding, compared with 9% within the group that didn’t take the treatment. Essentially the most common symptoms related to the bleeding was dizziness.
Overall, 14% of people that received lecanemab suffered serious antagonistic events within the clinical trial, compared with 11% of those that didn’t receive the treatment.
The authors of the study said longer clinical trials were needed to find out the efficacy and safety of lecanemab in patients with early Alzheimer’s disease.
The FDA said the prescribing information for lecanemab will include a warning a couple of risk of swelling and bleeding, broadly known as amyloid-related imaging abnormalities.
The death of a clinical trial participant within the Chicago area could also possibly be linked to lecanemab, in response to a research letter published within the Latest England Journal of Medicine this week.
The 65-year-old suffered a stroke and was hospitalized 4 days after their third lecanemab infusion. A CT scan performed after the patient’s stroke found extensive bleeding within the brain. An MRI performed 81 days before the stroke had not found any bleeding.
The patient had also received a drugs, called t-PA, used to interrupt apart blood clots that cause strokes. But extensive brain bleeding could be an unusual complication of this medication alone, in response to the physicians who penned the research letter.
Researchers involved within the lecanemab clinical trial, in a response letter, argued that the blood clot medication gave the impression to be the immediate reason behind the patient’s death, with the primary symptoms occurring 8 minutes after they received an infusion of the blood-clot buster.










